AQUIOS CL
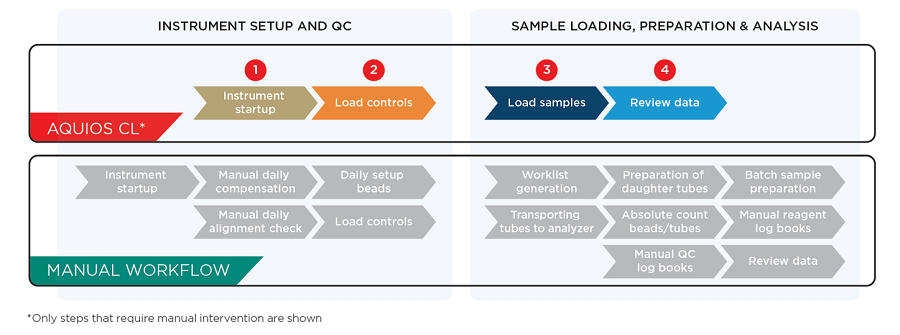
AQUIOS CL是首款真正全自動化(Load & Go)流式細胞儀,將樣品製備和分析結合在同一個平台中,同時優化了有限實驗室空間的使用。借助需要較少培訓的直觀技術,它可以提高生產率,同時減少容易出錯的步驟。
- 免拔蓋穿刺取樣改善潛在生物危害性樣品材料的處理
- 通過高通量效能提高生產率
- 通過條碼試劑和 LIS 連接實現完全可追溯性
- 相容於 Windows 10 作業系統
Automate Your Workflow
True load and go workflow automation on the AQUIOS CL allows you to run standardized IVD cleared assays for lymphocyte subset analysis. AQUIOS Designer Software (ADS) 2.0 additionally allows the usage of customized reagents to automate user-defined tests.
AQUIOS Tetra Reagents
With the AQUIOS Tetra Reagents you have an intuitive lymphocyte subset analysis. Running the analysis on the single platform technology of the AQUIOS CL allows the simultaneous identification and enumeration of T, B, and NK lymphocytes without the need for counting beads.
AQUIOS PLG
Using Pan Leukogating on the AQUIOS, the CD4 absolute count is directly derived from the fluorospheres run with the assay in a single platform. It only uses two antibodies while providing better population separation.
AQUIOS STEM System
The AQUIOS STEM System is the evolution of the gold standard for CD34 enumeration. The automation and traceability features of the AQUIOS CL provides the option to adapt panels to sample types while staying IVD compliant and remaining easy to use.
AQUIOS Designer Software
The AQUIOS Designer Software allows you to combine your user-defined assays with the Load & Go automation features of the AQUIOS CL. You can create user-defined tests, adapt sample preparation to your specific needs as well as implementing user-defined control material.
NOT ALL PRODUCTS ARE AVAILABLE IN ALL COUNTRIES.
PRODUCT AVAILABILITY AND REGULATORY STATUS DEPENDS ON COUNTRY REGISTRATION PER APPLICABLE REGULATIONS. To check if the product is available in your country, please contact your local sales representative or create a request.