Error Prevention During Quality Control on the AQUIOS CL System
The AQUIOS CL system employs several features that work to prevent errors during quality control. These prevention methods can be broken down into the following:
Table 4. Error Prevention Quality Control at a Glance
Automatically Pause when QC Fails
Refer to Laboratory SOP: Pause for QC in the Daily Quality Control on the AQUIOS CL System for the Tetra Application product bulletin for more about the Pause for QC function.
QC Checks
Unlike traditional flow cytometers the AQUIOS CL flow cytometer does not require additional reagents such as fluorospheres to perform QC checks when running the Tetra application. The system’s advanced algorithm checks a variety of internal criteria and will simply display pass or fail. The AQUIOS relies on quantitative data to determine the pass/fail parameters. There are four categories of QC checks on the AQUIOS CL flow cytometer:
-
Daily QC (Controls)
Daily QC is performed to ensure optical, fluidics and electronic stability within the system are operating within the instrument specifications. When enabled, the system provides a reminder to run controls. Refer to the Daily Quality Control on the AQUIOS CL System for the Tetra Application Product Bulletin for a detailed look at the QC process on the AQUIOS CL flow cytometer.
-
Real Time QC (Patient Sample)
Real time QC for the Patient Sample automatically monitors several variables during a sample run including: separation quotient (SQ), mean channel, stable data rates, and compensation checks. Since there is no range established for a given sample, the system will not flag it. However, the system will display a generic notification if there are any inconsistencies.
-
Application-Specific Quality Checks (Patient Sample)
AQUIOS Tetra Application. The AQUIOS Tetra application consists of three tests: the Tetra 1 test (CD45-FITC/CD4-RD1/CD8-ECD/CD3-PC5), the Tetra-2+ test (CD45-FITC/(CD56+CD16+) RD1/CD19- ECD/CD3-PC5), and the Tetra Combo test (AQUIOS Tetra 1 test followed by AQUIOS Tetra 2+ test).
The AQUIOS Tetra 1 Panel reagent also functions as an analytic reliability check for a specimen by monitoring the Total CD3+ absolute count. The CD3+ Reliability Check is the sum of the percentages of CD3+CD4+ and CD3+CD8+ cells and should be within ±5% of the total percentage of CD3+5.
The combination of AQUIOS Tetra 2+ Panel monoclonal antibody reagents can function as a quality control check for a specimen in terms of total lymphocyte percentage determined using the following formula5:
Total lymphocyte percentage (%) = %CD3+ (T) lymphocytes + %CD19+ (B) lymphocytes + %CD3-/CD56+ and/or CD16+ (NK) lymphocytes
The combination of monoclonal antibody reagents in the Tetra Combo test can function as a quality control check for a specimen in terms of total lymphocyte percentage determined using the same formula described above.
CD3+ Intrapanel Check in the Tetra Combo test is the variability between AQUIOS Tetra 1 and AQUIOS Tetra 2+ for CD3+ and serves as an internal control. Differences between replicate CD3 percent positive results should be ±3.5%.
-
System Checks
The system automatically verifies the laser power and that the vacuum is acceptable before each sample is run. The system also verifies internal communications and that the hardware is functioning correctly at each step of the process. In addition, the system performs a fluidic check at the start of each run to ensure there is enough AQUIOS Sheath Solution available for the requested run.
With the AQUIOS CL flow cytometry system, QC checks associated with instrument performance essentially follow the same principles that are behind the fluorosphere-based QC checks performed by general purpose flow cytometers. The key difference is that the AQUIOS CL flow cytometer is designed to perform these QC checks automatically without the need for additional reagents. This helps to simplify the process making QC checks associated with the instrument quick and efficient.
Fill the form to download Workflow Comparison Study with AQUIOS CL Flow Cytometer

HIV Advanced Disease Management

HIV Advanced Disease Management Solutions
Monitoring CD4 lymphocyte counts is essential in providing critical information that impacts patient care. CD4 monitoring allows caregivers to know when the disease transforms and stage its progression so they can implement the most appropriate intervention..
Fast Throughput Subset Analysis with AQUIOS CL
In the clinical management of immune deficiency diseases, accurately counting the absolute cell numbers of leukocyte subsets and measuring the percentage of individual subtypes in blood is critical.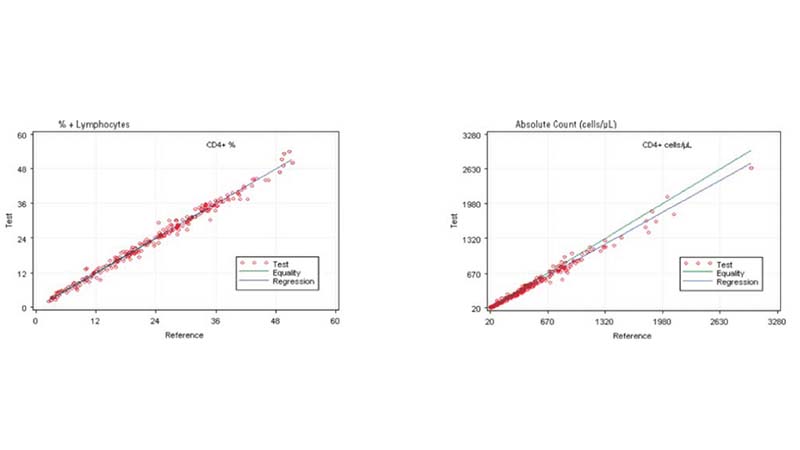
Comparing the AQUIOS CL PLG Application to the FC500 MCL FlowCARE PLG Application
Based on this study, the data suggests a strong correlation between the AQUIOS CL PLG application and the FC500 MCL FlowCARE PLG application.
AQUIOS CL Tetra and Aged Whole Blood samples
Based on this study, the AQUIOS CL instrument with Tetra application provides accurate results for recovery of the T, B and NK lymphocyte subsets in clinical and normal specimens collected into the EDTA K3 tubes when stored at room temperature for up to 24 hours.Error Prevention on AQUIOS CL overview
Laboratory standards and regulations set forth by governmental bodies such as the Centers for Disease Control (CDC) help to define the procedures that laboratories follow
Error prevention during Startup and Worklist creation
The AQUIOS CL system employs several features that work to prevent errors during startup, cleaning, and worklist generation, including Autocleaning, Test Requests to and from the LIS and Creating Worklists.
Error Prevention During Sample Prep
The AQUIOS CL system employs several features that work to prevent errors during sample prep.
Error Prevention During Quality Control
The AQUIOS CL system employs several features that work to prevent errors during quality control, including Automatically Pause when QC Fails, QC Checks, Daily QC (Controls), Real Time QC (Patient Sample).References:
- 1997 Revised Guidelines for the Performance of CD4+ T-Cell Determination in Persons with Human Immunodeficiency Virus (HIV) Infection. MMWR, January 10, 1997, p. 11.
- Levey S., Jennings, E.R., (1950) The use of control charts in the clinical laboratory. Am. J. Clin. Pathol. 20:1059.
- Hulspas R, Dombkowski D, Preffer F, Douglas D, Kildew-Shah B, Gilbert J. Flow Cytometry and the Stability of Phycoerythrin-Tandem Dye Conjugates. International Society for Advancement of Cytometry. September 23, 2009.
- Hulspas R, O’Gorman MRG, Wood BL, Gratama, JW, Sutherland DR. Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry Part B 2009;76B:355-364.
- Centers for Disease Control and Prevention. 2003 Guidelines for performing single-platform absolute CD4+ T-cell determinations with CD45 gating for persons infected with Human Immunodeficiency Virus HIV). MMWR 2003; 57 (No. RR. 2): 1-13.

